Introduction: CARTITUDE-2 (NCT04133636) is a phase 2, multicohort study evaluating the safety and efficacy of ciltacabtagene autoleucel (cilta-cel), an anti-BCMA chimeric antigen receptor (CAR)-T cell therapy, in various populations of patients with multiple myeloma (MM). We previously reported 17-month median follow-up results from cohort A (1-3 prior lines of therapy [LOT] and lenalidomide [len]-refractory) and 18-month median follow-up results from cohort B (early relapse: ≤12 months after either autologous stem cell transplant [ASCT] or start of initial anti-myeloma treatment, if not transplanted). Cilta-cel is also under evaluation in patients with len-refractory MM after 1-3 LOT in the phase 3 CARTITUDE-4 study, which showed cilta-cel significantly prolonged progression-free survival (PFS) vs standard of care (HR, 0.26) at a median follow-up of 16 months. Here, we present updated efficacy and safety data from CARTITUDE-2 cohorts A and B, both with a median follow-up of ~29 months.
Methods: Patients in cohorts A and B, all naive to CAR-T and/or anti-BCMA therapies, received a single cilta-cel infusion (target dose 0.75×10 6 CAR+ viable T cells/kg) 5-7 days after lymphodepletion. In both cohorts, the primary endpoint was minimal residual disease (MRD)-negativity (10 -5 threshold, by next-generation sequencing or next-generation flow cytometry). Management strategies were implemented after the phase 1b/2 CARTITUDE-1 study to reduce risk of movement and neurocognitive treatment-emergent adverse events (MNTs).
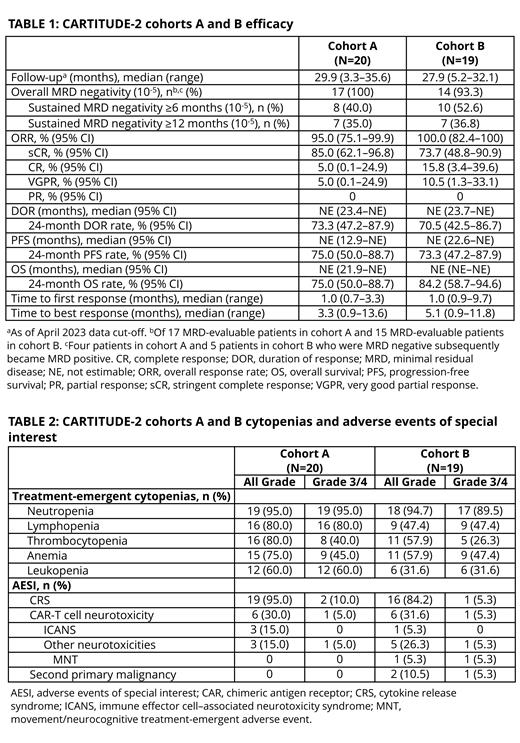
Results: As of April 2023, 20 patients in cohort A had received cilta-cel (median follow-up, 29.9 months; 35% with high-risk cytogenetics; median 2 prior LOT; 95% refractory to last LOT; 40% triple-class refractory; 85% with prior ASCT). At the same data cut-off, 19 patients in cohort B hadreceived cilta-cel (median follow-up, 27.9 months; 16% with high-risk cytogenetics; 79% refractory to last LOT; 16% triple-class refractory; 79% with prior ASCT). All (100%) 17 MRD-evaluable patients in cohort A and 14 (93%) of 15 MRD-evaluable patients in cohort B achieved MRD negativity (10 -5 threshold). Eight (40%) of 20 patients in cohort A and 10 (53%) of 19 patients in cohort B sustained MRD negativity at 10 -5 for ≥6 months (Table 1). In the 20 patients in cohort A and 19 in cohort B, cilta-cel led to overall response rates of 95% (complete response or better [≥CR], 90%) and 100% (≥CR, 90%), respectively. Median PFS was not reached in either cohort, and 24-month PFS rates were 75% in cohort A and 73% in cohort B; respective 24-month overall survival rates were 75% and 84%. In cohort A, hematologic treatment-emergent adverse events (TEAEs) occurring between 17.1- and 29.9-month median follow-up included maximum grade (gr) 3/4 leukopenia in 1 patient (all gr,12 total; 60%), maximum gr 3/4 lymphopenia in 2 patients (all gr,16 total; 80%), and maximum gr 3/4 thrombocytopenia in 1 patient (all gr,16 total; 80%). In cohort B, no new patients reported hematologic TEAEs between 18.0- and 27.9-month median follow-up (Table 2). In cohort A, no new patients had CAR-T cell neurotoxicity, and no patients had a second primary malignancy (SPM). In cohort B, no new patients had MNTs, but other neurotoxicity (gr 2 sensory loss) occurred in 1 additional patient (all gr, 5 total; 26%) and resolved; and SPM (gr 4 choroid melanoma) occurred in 1 additional patient (all gr, 2 total; 11%). One new death (total 5) occurred in cohort A on day 666 due to progressive disease, and 1 new death (total 4) occurred in cohort B on day 749 due to cardiac arrest (not treatment related).
Conclusions: These longer-term follow-up data show that patients treated with cilta-cel in earlier LOT, both those with len-refractory MM after 1-3 LOT (cohort A) and those with early relapse (cohort B), experienced deep and durable responses. No new CAR-T-related safety signals, except for 1 additional CAR-T cell neurotoxicity in cohort B, were reported. Cohort A provides insight into potential longer-term survival outcomes that may be expected in the phase 3 CARTITUDE-4 trial, which enrolled the same patient population but has shorter follow-up thus far. The long-term cohort B data highlight the durable efficacy of cilta-cel in patients who had early relapse; this is a functionally high-risk population for whom standard risk factors, including a high-risk cytogenetic profile, may not predict risk of relapse and for whom there is significant unmet need.
Disclosures
Hillengass:Prothena: Consultancy; Amgen: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Beigene: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; ESMO Florida: Other: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Targeted Oncology: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Angitia: Consultancy; Janssen: Consultancy, Other: DSMB; OncLive: Consultancy; Axxess Network: Consultancy; Sanofi: Consultancy; Oncopeptides: Consultancy; Skyline: Consultancy; GSK: Consultancy, Research Funding. Cohen:Abbvie: Consultancy; Janssen: Consultancy, Research Funding; GSK: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; Pfizer: Consultancy; BMS/Celgene: Consultancy; Ichnos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Patents & Royalties, Research Funding; Arcellx: Consultancy. Agha:GenCART, Inc.: Current equity holder in private company. Delforge:Sanofi: Consultancy, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Speakers Bureau; Stemline: Consultancy, Speakers Bureau. Roeloffzen:Janssen: Other: Travel grants, honoraria or advisory board (not personal); Bristol Myers Squibb: Other: Travel grants, honoraria or advisory board (not personal); AbbVie: Other: Travel grants, honoraria or advisory board (not personal); Sanofi: Other: Travel grants, honoraria or advisory board (not personal); Amgen: Other: Travel grants, honoraria or advisory board (not personal). Einsele:Amgen: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding; Janssen: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding; GlaxoSmithKline: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding; Novartis: Honoraria, Other: Consulting or advisory role, Travel support; Takeda: Honoraria, Other: Consulting or advisory role, Travel support; Sanofi: Honoraria, Other: Consulting or advisory role, Travel support, Research Funding. Goldschmidt:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Mundipharma: Research Funding; Array Biopharma: Research Funding; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Chugai: Honoraria, Patents & Royalties, Research Funding; Dietmar-Hopp-Foundation: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Johns Hopkins University: Research Funding; Hoffman- La Roche: Research Funding; KaryoPharm: Research Funding; Molecular Partners: Research Funding; MSD: Research Funding; Pfizer: Honoraria, Patents & Royalties: Travel Support, Research Funding; Morphosys AG: Research Funding; GSK: Honoraria, Other: Travel Support, Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Glycomimetics: Research Funding; Millenium Pharmaceuticals: Research Funding; Incyte: Research Funding; Heidelberg Pharma: Research Funding; Takeda: Research Funding; Novartis: Honoraria, Other: Travel Support, Research Funding; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees. Weisel:Adaptive Biotech: Consultancy, Honoraria; Stemline: Honoraria; AstraZeneca: Honoraria; Roche Pharma: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Research grant to institution; Bristol Myers Squibb/Celgene: Consultancy, Honoraria, Other: Research grant to institution; Takeda: Consultancy, Honoraria, Other: Research grant; Karyopharm: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Other: Research grant to institution; Novartis: Honoraria; GlaxoSmithKline: Consultancy, Honoraria, Other: Research grant to institution; Oncopeptides: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; Menarini: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Other: Research grant to institution; AbbVie: Consultancy, Honoraria, Other: Research grant to institution. Raab:Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Heidelberg Pharma: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Heidelberg University Hospital: Current Employment; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Sonneveld:Pfizer: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Zweegman:Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding. Schecter:Janssen: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties: Janssen. De Braganca:Janssen: Current Employment. Jackson:Janssen R&D: Current Employment, Current equity holder in publicly-traded company. Vlummens:Janssen Oncology/JnJ: Current Employment. Varsos:Janssen R&D: Current Employment, Current equity holder in private company. Corsale:Janssen: Current Employment, Current equity holder in publicly-traded company. Madduri:Janssen R&D: Current Employment. Yeh:Janssen R&D: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Roccia:Janssen: Current Employment, Current equity holder in private company. Song:Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company. Akram:Legend Biotech: Current Employment, Current equity holder in publicly-traded company. Costa Filho:Legend Biotech: Current Employment, Current equity holder in publicly-traded company. Geng:Legend Biotech: Current Employment. Cohen:GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Van De Donk:Janssen Pharmaceuticals, Amgen, Celgene, Novartis, Cellectis, BMS: Research Funding; Janssen, Amgen, Celgene, BMS, Takeda, Roche, Novartis, Bayer, Adaptive, Servier: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal